2022-03-09 2162
On November 26, as reviewed by the NMPA, the registration of Johnson&Johnson Vision Catalys precision laser system was approved, which was the second innovative medical product to be approved based on a clinical evaluation assisted with real-world evidence after the drainage tube for glaucoma and signified a new breakthrough achieved in the real-world data pilot programs in Lecheng.
Johnson&Johnson Catalys precision laser system is an integrated scanning laser system, used for removing crystalline lens in the cataract surgery, including the lens anterior capsule section surgery, crystalline lens crushing surgery, in-cornea single plane and multi-plane arc cutting/cut production. The system boasts high accuracy and precision and a safer performance, which can customize the setting for cornea incision and cut automatically with an accuracy up to 1/4 of a hair. It can minimize the injury to cornea, iris and posterior capsule, achieve more efficient, accurate and comfortable surgery operation, and bring a clear cornea, a better eyesight and a better prognosis result for the patients.

As one of the first batch of pilot products for real-world study programs of Lecheng Pilot Zone, the research project is a foresighted, single-group and observational clinical research conducted on the precision laser system in actual clinical diagnosis and treatment, which is intended to evaluate the overall performance of the system.
Embarking on the fast track of the piloting policy of Lecheng, the “Catalys” precision laser system has achieved positive results in the treatment of Chinese cataract patients. After the first surgery was finished successfully at Boao Super Hospital in November 2019, over 100 cataract patients have benefited from the innovative medical device through real-world study programs.
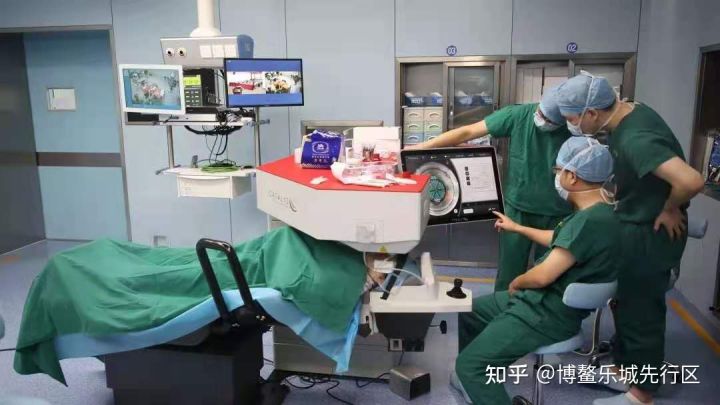
The Catalys precision laser system was the first medical device introduced by Lecheng Pilot Zone. The approval this time was an important milestone of real-world data pilot programs in Lecheng Pilot Zone, and the second product approved with real-world study at Lecheng after the registration of the US Allergan drainage tube for glaucoma approved by the NMPA on March 26. It will provide the “experience of Hainan” for accelerating the accessibility of global innovative medical devices in China.
Professor Chen Wei, director of the Eye Center of Boao Super Hospital and the person in charge of real-world study of Catalys femtosecond cataract surgery, said, Lecheng will become a window for global innovative eye technologies to enter China. The marketing of the product has brought good news to the cataract patients, who will be able to enjoy international advanced medical products without going abroad. Supported by these favorable policies, the Eye Center of Boao Super Hospital will try to build a “window for global innovative eye technologies into China” and an “innovation platform for the R&D, application and commercialization of cutting-edge international eye medical products”.
“This is an important innovative practice of the NMPA in the regulatory science as well as a solid step taken towards the reform of national medical products approval system,” Gu Gang, director of Lecheng Pilot Zone Administration, said, the approval and marketing of the product was a new fruit in the construction of Hainan Free Trade Port supported by the NMPA and more a vivid reflection of the institutional integration and innovation promoted by several departments together. The fruit will attract more international advanced medical product manufacturers and medical institutions to settle in Lecheng. In the future, we will go all out to promote the construction of the NMPA Medical Products Regulatory Science Research Base, plan and prepare for the Real-world Data and Evidence Study Conference, and contribute to promoting China to be an institutional and technological authority in the global real-world studies.