2021-03-28 273
Hot search topics on March 28↓↓↓
#Lecheng Medical Special Zone#
#Approval of new drug using real world data from Lecheng#
Ranked third on Weibo's hot search list
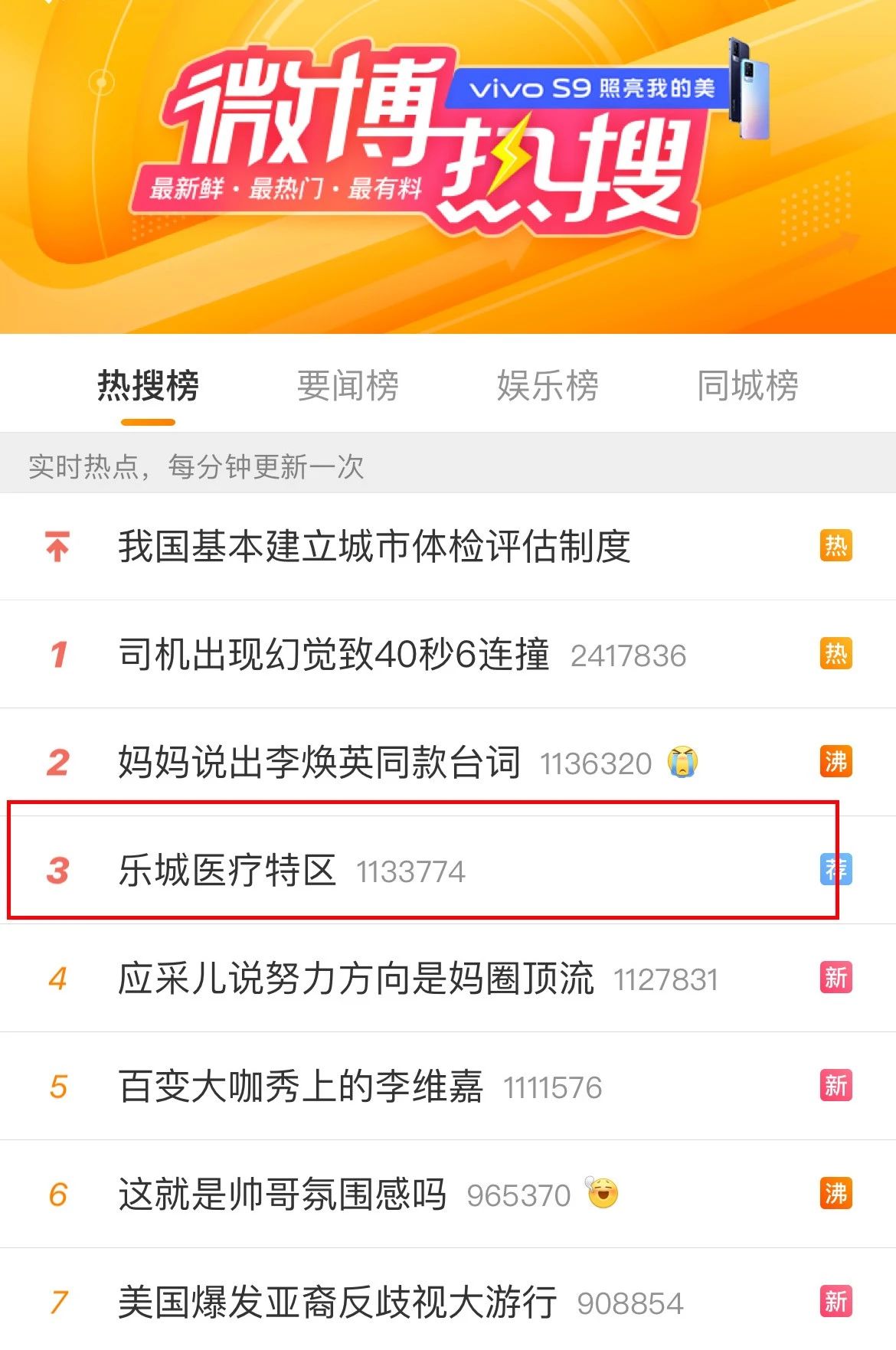
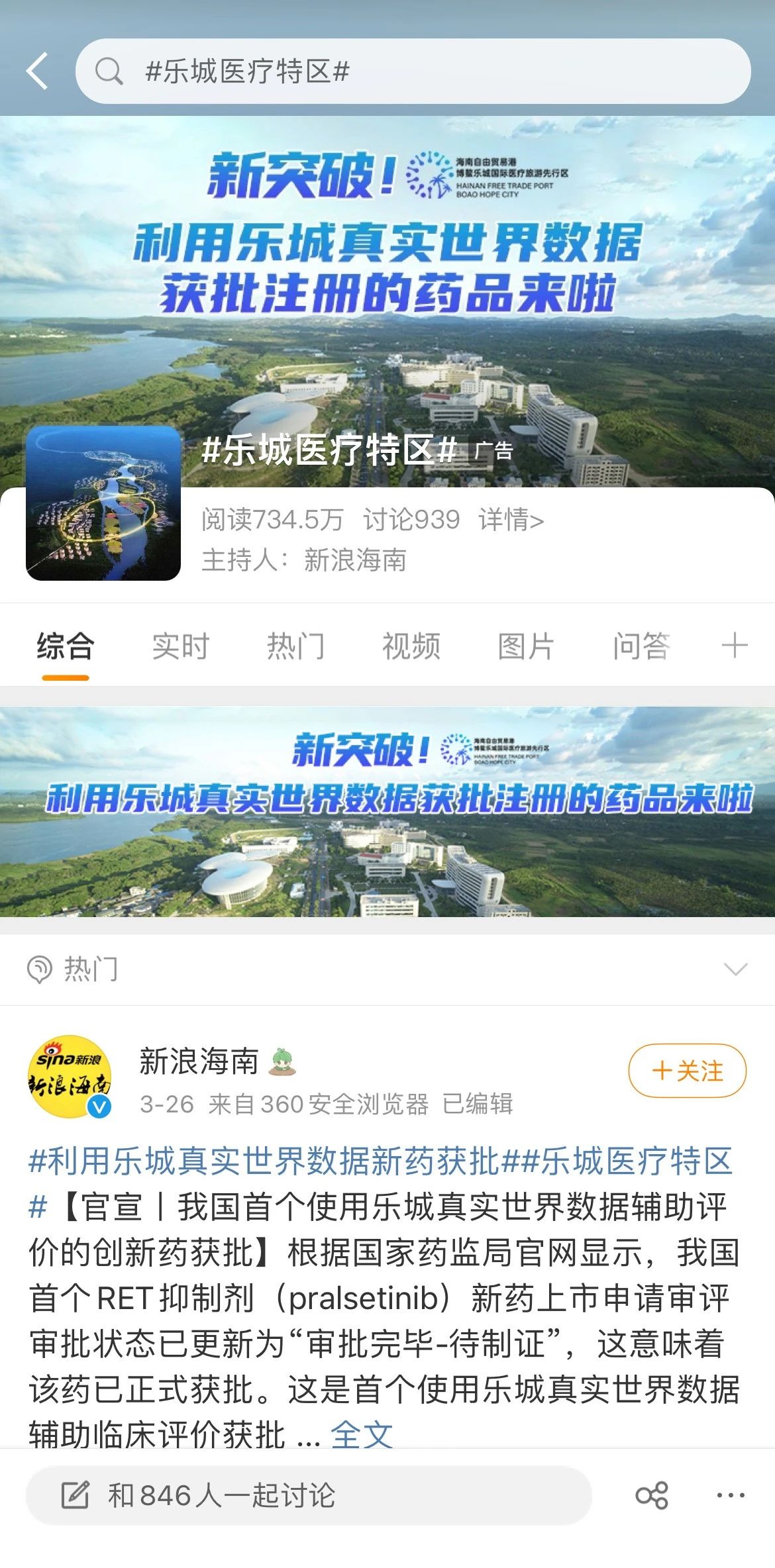
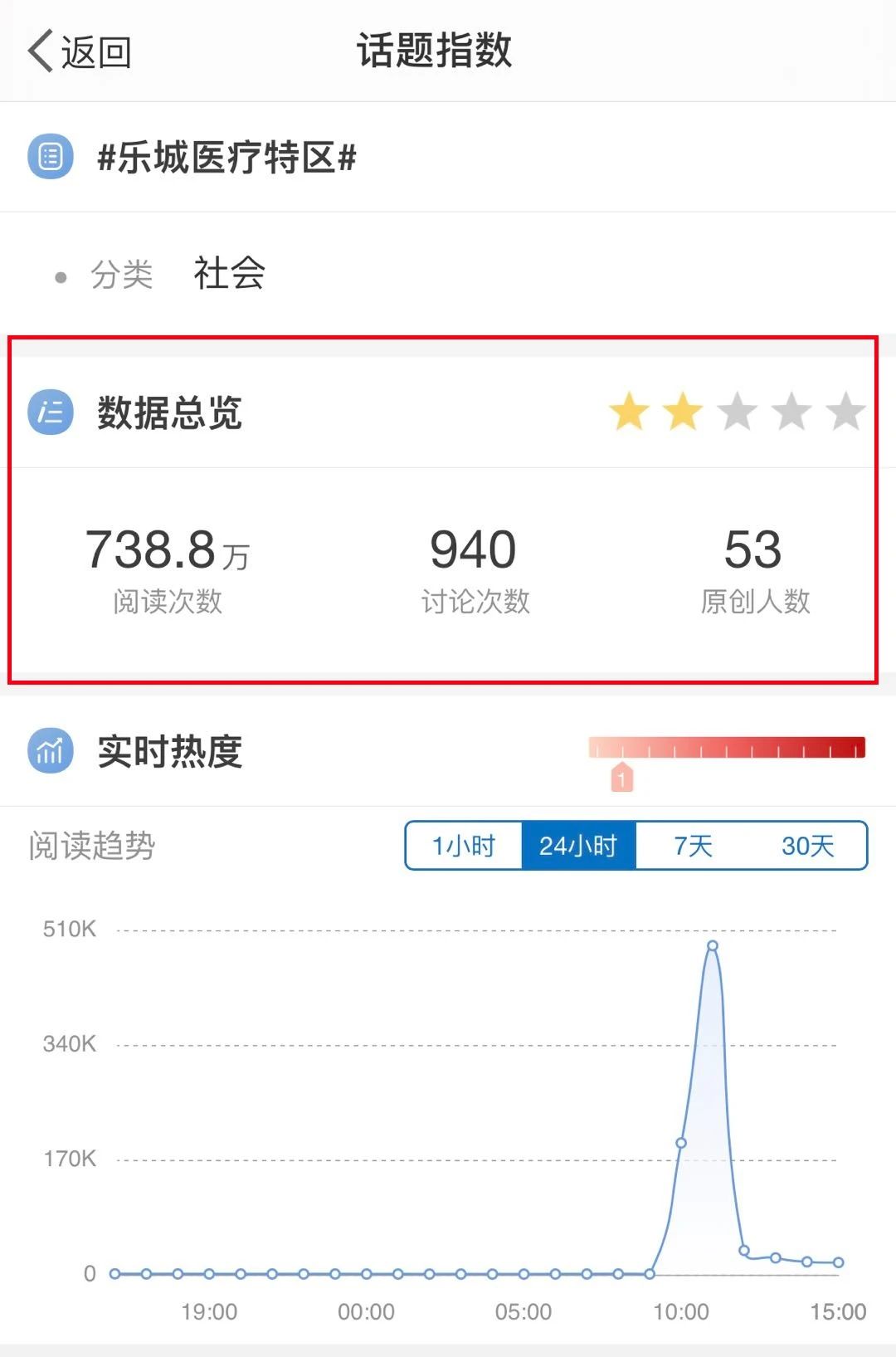
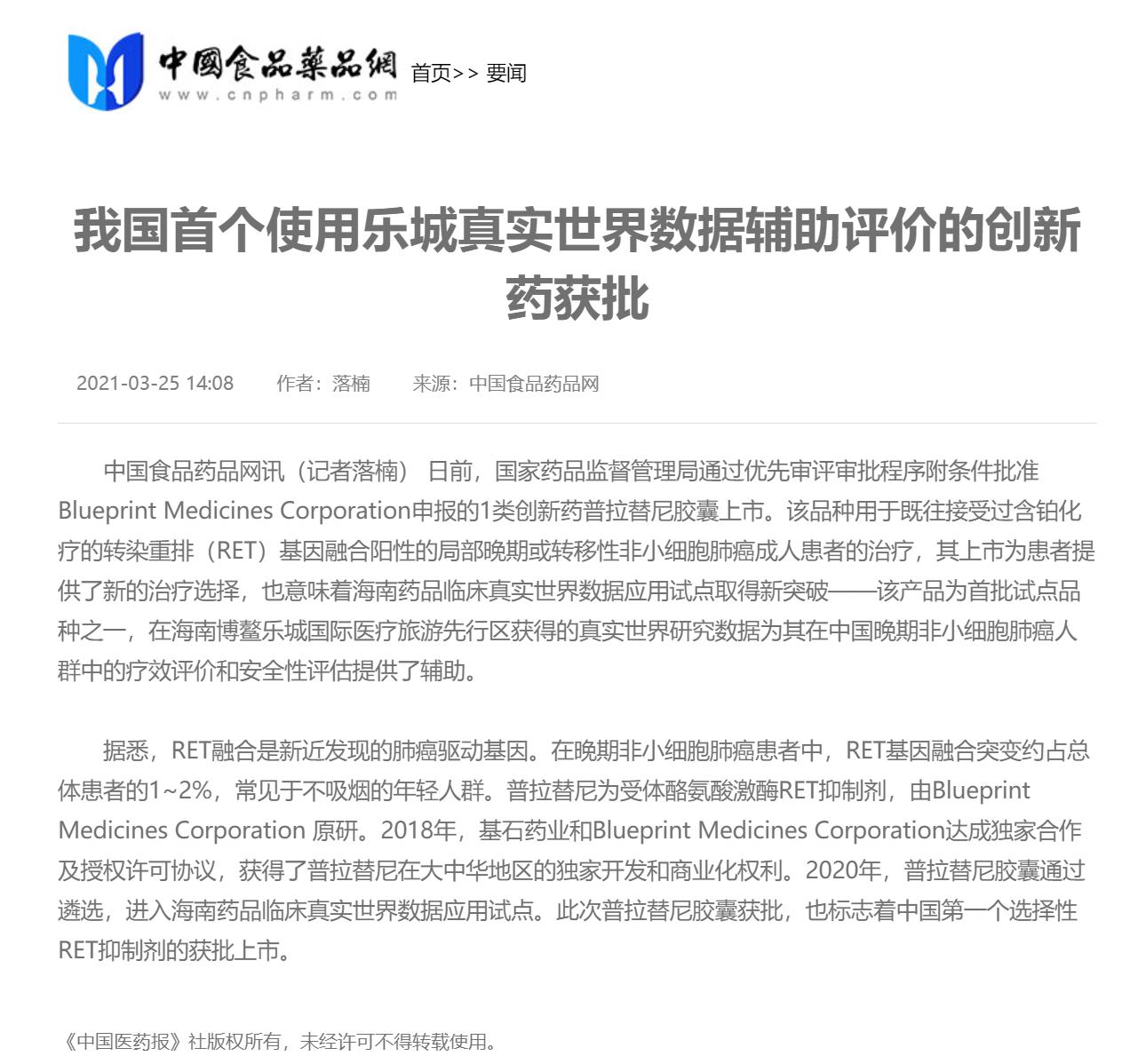
On March 25, according to the official website of the State Food and Drug Administration, the review and approval status of my country's first RET inhibitor (pralsetinib) new drug marketing application has been updated to "approval completed - pending certification", which means that the drug has been officially approved. . This is the first approved drug that uses Lecheng real-world data to assist clinical evaluation, marking a major breakthrough in the pilot application of clinical real-world data for Boao Lecheng drugs in Hainan.
This wave of "hard core" operations
Let the majority of netizens praise
Everyone also retweeted




2021 is the 100th anniversary of the founding of the party and the first year of the "14th Five-Year Plan". It is a key year for Hainan to comprehensively deepen reform and opening up and accelerate the construction of a free trade port. It is also a very important year for the Lecheng Pioneer District to achieve high-quality development. The Lecheng Pioneer District will base itself on the new development stage, implement the new development concept, further emancipate the mind, dare to try, and innovate boldly, and based on "allowing Chinese patients to use the latest international medical devices", to promote international advanced medical resources and domestic health needs Effectively connect, cultivate the national drug and medical device review and approval system and the "experimental field" of regulatory reform, and promote the application of my country's clinical real data to seize the "world first".