2022-01-01 303
The pilot work on the application of clinical real-world data for drugs and medical devices is one of the major institutional innovations in Hainan Province and an important part of China's scientific action plan for drug regulation. In 2021, under the careful guidance of the State Food and Drug Administration, under the strong leadership of the Hainan Provincial Party Committee and the Provincial Government, and with the strong support of the Hainan Provincial Food and Drug Administration and the Hainan Provincial Health and Health Commission, the Lecheng Administration will give full play to the State Food and Drug Administration. The platform advantages of the Bureau's Drug Device Supervision Scientific Research Base (Hainan Real World Data Research Institute) and the "experimental field" role of the Lecheng Pioneer District, vigorously introduce international innovative drug device products, promote the pilot work of clinical real world data application, and actively explore Use clinical real-world data for drug and medical device product registration and regulatory decision-making practices.
Femtosecond laser ophthalmic treatment system and pratinib, two licensed pharmaceutical and device products, were approved for marketing with the help of Lecheng real-world research; tralaciride for injection, fluocinolone vitreous implant, cochlear implant, thermal ablation system, The registration applications of 5 licensed medicinal and device products including the cochlear implant sound processor have entered the review stage... This year, the pilot work of clinical real-world data application has made continuous breakthroughs, providing new insights for the reform of the national drug and medical device review and approval system. Ways and methods to speed up the registration and listing of international innovative pharmaceutical products in China to better meet the needs of public health.
Pilot work is advancing
The State Food and Drug Administration and the Hainan Provincial Government jointly promote the pilot work related to the application of clinical real-world data for medical devices
On December 28th, the State Food and Drug Administration and the Hainan Provincial Government jointly held the third meeting of the leading group for the pilot work of clinical real-world data application for drugs and medical devices in 2021, summarizing the experience of clinical real-world data application pilot work, and discussing in-depth promotion of real-world data application. Research new measures to help the high-quality development of the biopharmaceutical industry in Hainan Free Trade Port and the reform of the review and approval system for drugs and medical devices. Wang Bin, vice governor of Hainan Province, and Xu Jinghe, deputy director of the State Food and Drug Administration, attended the meeting and delivered speeches.




The first drug that uses Lecheng real-world data to assist clinical evaluation is approved
In March 2021, the State Food and Drug Administration approved the listing of the Class 1 innovative drug Pratinib Capsules declared by Blueprint Medicines Corporation with conditions through the priority review and approval process. This product is also a pilot product for the application of clinical real-world data in Hainan. The relevant real-world research results are used as a supplement to the clinical trial results, and provide assistance for its efficacy evaluation and safety evaluation in Chinese patients with advanced non-small cell lung cancer. This is the first approved drug that uses Lecheng real-world data to assist clinical evaluation, marking a major breakthrough in the pilot application of clinical real-world data for Boao Lecheng drugs in Hainan.

Johnson & Johnson's Catalys femtosecond laser eye treatment system is approved for registration using Lecheng real-world data
On January 26, after review by the State Drug Administration, the registration of Johnson & Johnson's Catalys femtosecond laser eye treatment system was approved. This is the second case of an innovative medical device product that has been approved for clinical evaluation using Lecheng Clinical real-world evidence after the glaucoma drainage tube.

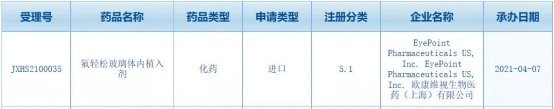
China's first new drug OT-401 (Yutiq) to declare NDA based entirely on Lecheng real-world research data was accepted by NMPA
On April 7, 2021, Oconverse announced that the New Drug Application (NDA) of its pipeline core product OT-401 (fluocinolone vitreous implant) was officially accepted by the State Food and Drug Administration. The new drug for which the world data declared NDA is also the first time that the State Food and Drug Administration has accepted the drug NDA declared by the real world data of Lecheng.
The application for registration and marketing authorization of tralacilide for injection was accepted by the State Food and Drug Administration
On November 29, 2021, the State Drug Administration accepted the application for registration and marketing authorization for overseas production of trilaciclib for injection submitted by Simcere Pharmaceuticals (English generic name Trilaciclib). Prophylactic use of glycoside regimen in patients with extensive-stage small cell lung cancer (ES-SCLC) to reduce the incidence of chemotherapy-induced myelosuppression, marking a new achievement in the Lecheng clinical real-world data application pilot work.
Bleak

The first communication meeting in 2021 for the pilot work of real-world data application of Hainan Pharmaceuticals was held
On July 15, the first communication meeting in 2021 for the pilot work of real-world data application of Hainan Pharmaceuticals was held in Haikou, Hainan. The purpose of the meeting is to strengthen communication and exchanges with enterprises and medical institutions of real-world research drug pilot varieties, to study and discuss the problems and countermeasures existing in the research of pilot varieties, to discuss the paths and measures to carry out real-world research on children's drugs and traditional Chinese medicine, and to further promote real-world research. Research work is carried out.


Zhou Siyuan Deputy Director


Minister Yang Zhimin Deputy Minister Wang Jun
Lecheng Pharmaceutical Real World Research Variety Communication Meeting Held
On July 16, the Drug Evaluation Center of the State Food and Drug Administration, the Hainan Provincial Food and Drug Administration, and the Boao Lecheng Pilot Area Administration held a communication meeting on real-world research varieties of drugs in Haikou to communicate with companies that plan to conduct real-world research. Discuss the scientificity and feasibility of real-world research plans for related drug varieties and the availability of subsequent registration and declaration of products using Lecheng real-world data to assist with product registration. The products communicated involve oncology, endocrinology, ophthalmology and other fields.


The Drug Evaluation Center of the State Food and Drug Administration held a real-world seminar on children's drug use
In order to actively implement the relevant requirements of the State Council on children's drugs, on July 15, the Drug Evaluation Center of the State Drug Administration, the Hainan Provincial Drug Administration, the Boao Lecheng Administration, clinical and statistical experts and some children's drug companies held a special topic in Haikou. Conference to discuss the use of real-world research to address issues related to the evidence on medication use in children. The meeting provided an extensive exchange of views on the use of real-world research to support and provide evidence for the use of medicines in children, to expand the indications of medicines in children, research methods and concerns.

The Drug Evaluation Center of the State Food and Drug Administration held a seminar on real-world research on traditional Chinese medicine
In order to promote the real-world research of traditional Chinese medicine, the Center for Drug Evaluation of the State Drug Administration, together with the Hainan Provincial Drug Administration, the Boao Lecheng Administration Bureau and the Hainan Provincial Real-World Data Research Institute, held a meeting of traditional Chinese medicine in Haikou on the afternoon of July 15, 2021. Real World Research Symposium. The meeting mainly discussed the problems, difficulties and challenges existing in the real-world research of traditional Chinese medicine, and clarified the direction and measures for the next step.


The Provincial Food and Drug Administration held a symposium on the pilot varieties of medical device clinical real-world research and application in Lecheng
In order to further promote the implementation and development of the pilot variety plan for clinical real-world data application, and to standardize and accelerate the clinical trial process, on February 3, the Hainan Provincial Drug Administration held a symposium on pilot varieties of clinical real-world research and application of medical devices in Lecheng Pioneer Area .


Supervising the construction of scientific bases
China's first key laboratory for real-world data research and evaluation settled in Lecheng
In February 2021, the State Drug Administration issued the "Notice on the Accreditation of the Second Batch of Key Laboratories", and the Hainan Key Laboratory of Real World Data Research and Evaluation was recognized by the State Drug Administration, the country's first real world data research and evaluation. Key laboratory settled in Hainan.
This key laboratory is jointly built, managed and shared by three units, West China Hospital of Sichuan University, Boao Super Hospital, and the Adverse Drug Reaction Monitoring Center of Boao Pilot Area of Hainan Province. Taking Boao Lecheng as the test field, it innovates the practice of real-world data research and forms a Hainan-friendly laboratory. The series of research results featuring the free trade port will play an important role in building a domestic leading and internationally advanced real-world data research system and cultivating and exporting high-level, multidisciplinary talent echelons in the future, and will help my country seize the field of clinical real-world data application "World No. 1" provides strong technical support.
In 2021, the work symposium on the scientific base for drug and medical device supervision of the State Food and Drug Administration of Hainan Province and the first working meeting of the president of the research institute will be held
In order to implement the spirit of the instructions of the State Food and Drug Administration and the Hainan Provincial Party Committee and Provincial Government, on March 21, the 2021 Hainan Provincial State Food and Drug Administration Drug and Medical Device Regulatory Scientific Research Base Work Symposium and the President of Hainan Real World Data Research Institute The first working meeting was held in the Boao Lecheng Pioneer Zone to discuss the construction plan of the regulatory science base and research institute, and contribute Hainan's wisdom and Lecheng strength to the development of my country's regulatory science and health undertakings.




The first postgraduate training center in China with the training goal of "real world data application"
As an emerging interdisciplinary field, real-world research involves clinical medicine, biostatistics, information and data and other disciplines. It is a postgraduate training point with the training goal of "real world data application". In 2021, 10 masters and 5 doctoral candidates will be jointly trained, and the enrollment target will be gradually expanded every year.
The team of the Hainan Key Laboratory of Real World Data Research and Evaluation of the State Food and Drug Administration has been supported by 3 projects of the National Natural Science Foundation of China
On August 18, the review results of the 2021 National Natural Science Foundation of China's application project were announced. The Hainan Key Laboratory of Real World Data Research and Evaluation of the State Food and Drug Administration (hereinafter referred to as the key laboratory) team was supported by 3 Natural Science Foundation projects. The three National Natural Science Foundation of China projects approved this time are all based on the real-world data system of Boao Lecheng Specialized Diseases carefully built by the key laboratory. Applications play a key role in boosting.
The preparatory meeting for the sub-forum of the first Boao International Conference on Real World Research on Pharmaceutical Devices was held in Beijing
In order to better advance the preparations for the 1st Boao International Conference on Real World Research on Pharmaceutical Devices and turn the conference into a high-level academic event for real world research, on October 13-14, the Lecheng Administration and the State Food and Drug Administration The International Exchange Center of the Bureau, Hainan Provincial Drug Administration, and China Health Media Group held a preparatory meeting for the True Research Conference in Beijing.



Six Specialized Diseases Real World Data Research Alliance Unveiled
On October 16, at the "Annual Meeting of the Hainan Key Laboratory of Real World Data Research and Evaluation of the State Food and Drug Administration and the Scientific Summit of Clinical Research, Evaluation and Transformation Supervision", Li Lanjuan, academician of the Chinese Academy of Engineering, Zheng Shusen, academician of the Chinese Academy of Engineering, and academician of the Chinese Academy of Engineering. Shang Hong, Qu Jia, President of Wenzhou Medical University Affiliated Ophthalmology Hospital, Li Weimin, President of West China Hospital, Sichuan University, and Cai Jianqiang, Deputy Director of National Cancer Center and Vice President of Cancer Hospital of Chinese Academy of Medical Sciences The real-world data research alliance of six specialties, including laboratory, ophthalmology, respiratory system disease and tumor disease, was inaugurated.

Boao Lecheng Clinical Research Center ESR Project Released
On October 26, the ESR project of Boao Lecheng Clinical Research Center was officially released. The project is jointly developed by Yao Chen, Vice President of Hainan Real World Data Research Institute, Boao Lecheng Clinical Research Center, and Hangzhou Laimai Medical Information Technology Co., Ltd. The various real-world research projects in China provide high-quality source data, which is expected to solve the bottleneck problem that restricts my country's imported licensed pharmaceutical equipment project to carry out real-world research in the Lecheng Pilot Area.


Hainan Key Laboratory of Real World Data Publishes Methodological Articles of Chinese Medicine Efficacy Evaluation
In Frontiers in Pharmacology (IF=5.81, JCR Zone 1), the research team from the China Center for Evidence-Based Medicine, West China Hospital of Sichuan University / Hainan Key Laboratory of Real World Data Research and Evaluation of the State Food and Drug Administration, published a report entitled "Assessing clinical effects of traditional Chinese Medicine interventions: moving beyond randomized controlled trials". This article breaks through the traditional thinking of evaluating the efficacy of traditional Chinese medicine based on traditional experiments, and establishes an integrated innovation model of traditional experiments + real-world data research. The practice of medicine provides a degree of methodological technical preparation.

Published a special issue of "Real-World Data Research and Drug Device Regulatory Innovation"
In the 11th issue of 2021, "China Food and Drug Administration" magazine and Hainan Real World Data Research Institute jointly established a special issue of "Real World Data Research and Drug Device Supervision Innovation" and published, introducing real world data research design, data governance technology and statistical analysis methods Research progress, summarizes the real-world evidence to support the current status of global medical device regulatory decision-making, displays real-world data research results and cases in Hainan Boao Lecheng International Medical Tourism Pilot Zone, demonstrates the advantages of system integration and innovation with high-quality academic construction, and serves public health. .

Real research platform construction
The consultation meeting for the preliminary design and budget proposal of the Hainan Provincial Clinical Real World Data Research Platform Project was held in Beijing
In order to further promote the pilot work of Boao Lecheng clinical real-world data application and accelerate the construction of the "Hainan Provincial Clinical Real-world Data Research Platform Project", on the morning of February 25, the Lecheng Pilot Area Administration and the Hainan Provincial Drug Administration jointly held a meeting in Beijing. Held a consultation meeting on the preliminary design and budget estimates of the Hainan Province Real World Data Research Platform Project. The meeting conducted research and discussion on project requirements, corresponding functional content, composition of budget estimates, and issues that need to be clarified, and proposed solutions, and basically reached consensus on construction ideas and functional sections.


The Lecheng Administration held the second consultation meeting on the preliminary design and budgetary plan of the clinical real-world data research platform project
In order to improve the scientificity and feasibility of the construction of the "Hainan Province Clinical Real World Data Research Platform Project", on March 2, the Lecheng Pioneer District Administration held the preliminary design and budget estimates for the Hainan Province Real World Data Research Platform (Phase I) project. In the second opinion consultation meeting, the main responsible persons of medical institutions in the district, information management personnel, and relevant personnel of CRO enterprises were solicited opinions on the plan design.

Zhenyan platform function display and consultation meeting held
After half a year of construction, the core function development of six major business modules, including the Hainan Provincial Clinical Real World Data Research Platform, the Data Collection and Governance Platform, has been basically completed. In order to ensure the scientificity and operability of the real research platform, on the afternoon of October 14, the Hainan Real World Data Research Institute organized a function demonstration and consultation meeting of the real research platform in Beijing. Expert representatives attending the meeting fully affirmed the construction of the platform, believing that the platform has four characteristics of mechanism innovation, data innovation, supervision innovation and service innovation, and put forward constructive opinions on the aspects that the platform needs to be further improved.
State Food and Drug Administration Information Center, Drug Evaluation Center, Medical Device Technology Evaluation Center, Hainan Provincial Food and Drug Administration, Hainan Provincial Big Data Bureau, Lecheng Administration, Hainan Provincial Real World Data Research Institute, as well as pharmaceutical equipment companies, CRO companies and other relevant responsible persons to participate in the meeting through online and offline methods.


Academic exchange and training
"National team" experts lead, precise efforts, Lecheng real world data application opens a new stage
On the afternoon of April 12, at the 2020 International Innovative Pharmaceutical Equipment Work Summary Conference in Lecheng Pioneer District, Mao Zhenbin, Director of the Science and Technology and International Cooperation Department of the State Food and Drug Administration, Yang Zhimin, Director of the Drug Evaluation Center of the State Food and Drug Administration, and Medical Liu Yinghui, director of the Center for Device Evaluation, delivered keynote reports respectively.

Mao Zhenbin, first-level inspector , Minister Yang Zhimin, Minister Liu Yinghui
The annual meeting of the Hainan Key Laboratory of Real World Data Research and Evaluation of the State Food and Drug Administration and the Clinical Research, Evaluation and Translational Regulatory Science Summit opened
On October 16, 2021, the "Annual Meeting of the Hainan Key Laboratory of Real World Data Research and Evaluation of the State Food and Drug Administration and the Scientific Summit of Clinical Research, Evaluation and Translational Supervision" opened in Hainan. More than 400 experts and scholars from various fields participated on the spot to discuss the grand blueprint for the development of real-world data research. At the meeting, the "Key Laboratory of Hainan Real World Data Research and Evaluation of the State Food and Drug Administration" jointly established by West China Hospital of Sichuan University, Boao Super Hospital, and the Adverse Drug Reaction Monitoring Center of Hainan Boao Lecheng Pilot Area was officially completed.




Hainan Real World Data Research Institute Held Lecheng Pharmaceutical Real World Data Research Training and Symposium
In order to implement the strategic deployment of the State Drug Administration and the Hainan Provincial Party Committee and Government, and better serve the enterprises that are conducting or will conduct real-world research on licensed drugs in Lecheng, on February 28, sponsored by the Hainan Real-World Data Research Institute The pharmaceutical real-world data research training and symposium was held in the Boao Lecheng Pioneer Zone.




Medical device real world data research training meeting was held in Lecheng
On April 12th, the medical device real-world data research training meeting hosted by the Hainan Real-world Data Research Institute was held in the Boao Lecheng Pioneer Zone, aiming to further strengthen the policy of medical device companies to conduct real-world research in the Lecheng Pioneer Zone. , regulations, procedures, research environment and other in-depth understanding, and jointly promote the compliant and efficient development of real-world research in the Lecheng Pioneer Zone.

2021 Free Trade Port Real World Data Application Advanced Seminar Successfully Held in Boao
On June 7-9, 2021, the Advanced Training Institute of the State Food and Drug Administration and the Hainan Real World Data Research Institute successfully jointly held the 2021 Free Trade Port Real World Data Application Advanced Training Course in Boao, Hainan. This training focuses on learning relevant theoretical knowledge and practical experience. Through a combination of classroom lectures and practical exercises, a communication platform is built to help enterprises understand relevant policies and theoretical knowledge such as licensed pharmaceutical equipment in the park and real-world data applications.


2021 Clinical Research Lecheng Closed-door Seminar Held in Lecheng
On June 17, 2021, the Lecheng International Medical Tourism Pilot Zone Administration, the China Clinical Research Community, and the Hainan Real World Data Research Institute jointly held the "2021 Clinical Research Lecheng Closed-door Seminar" in the Lecheng Pilot Zone. The participants discussed in depth how to better gather the strengths of all parties, establish a benign interaction between the Lecheng Pioneer Zone and outside the province in terms of scientific research cooperation and industrial collaboration, and explore innovative clinical research models to promote the development of real-world research and help Promote the landing of international advanced medical equipment in the Hainan Lecheng Pilot Zone and other topics.


Real World Research Drug Clinical Research Training Course Held in Lecheng
On August 30, the real-world research drug clinical research training course jointly organized by the Hainan Real World Data Research Institute and the Boao Lecheng Clinical Research Center started in the Lecheng Pioneer Zone. More than 100 people from medical institutions, drug manufacturers, drug R&D institutions and CRO companies participated in the training online. This training aims to promote the construction of drug clinical trial institutions, strengthen the grasp of GCP and other knowledge of drug clinical research staff, ensure the smooth development of drug clinical research, cultivate first-class research teams and research hospitals, and accelerate the development of real-world research.

Real World Research Theory and Practice Training Course Held
On December 17, Hainan Real World Data Research Institute, Hainan Food and Drug Management Vocational Skills Training Center and Boao Lecheng Clinical Research Center jointly held the 2021 Real World Research Theory and Practice Training Course in Boao Lecheng. More than 200 people from enterprises, research institutions, and colleges and universities participated online and offline.

Outlook for 2022
The Lecheng Pioneer Zone will continue to give full play to the role of the "experimental field" as a pilot zone, guided by institutional innovation, and make good use of the licensed use of medicines and real-world data research policies granted to the Lecheng Pioneer Zone by the state. Adhere to systematic planning and resource planning, make every effort to hold the first Boao International Conference on Real World Research on Pharmaceutical Devices, and build an internationally authoritative clinical real world research platform. In-depth exploration of the supporting role of real-world research in my country's drug and medical device regulatory decision-making, striving for more innovative clinically urgently needed products to be approved for marketing faster, for the reform of China's drug and medical device review and approval system, and for accelerating the registration and approval of foreign innovative medical products in China Contribute to "Lecheng Plan" and "Hainan Power".