2022-03-23 808
translation of clinical research evidence into rapid recommendations for traditional Chinese medicine interventions: A methodological framework”的文章。
Recently, an article entitled “Toward better translation of clinical research evidence into rapid recommendations for traditional Chinese medicine interventions: A methodological framework” was published on the journal of Integrative Medicine Research by the research team of Chinese Cochrane Center of West China Hospital of Sichuan University and NMPA Key Laboratory for Real-World Data Research and Evaluation in Hainan.
The article was finished by related experts from Chinese Cochrane Center of West China Hospital of Sichuan University, Cochrane Center of Lanzhou University, Chengdu University of TCM, Jiangxi University of Chinese Medicine, Shanghai University of Traditional Chinese Medicine and Guangdong Provincial Hospital of Chinese Medicine, under the leadership of professor Sun Xin.
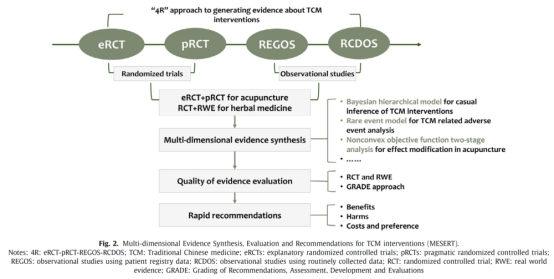
The development and application of clinical practice guidelines is a key path to translate clinical research evidence of TCM efficacy into diagnosis and treatment practice. However, due to the limitations such as the long production cycle and the inadequate utilization of TCM real-world evidence, the efficient translation of TCM efficacy evidence is still faced with challenges.
Based on the characteristics of TCM evidence, the international progress of guideline production methodology, and the “4R” path of TCM clinical efficacy evaluation, this article innovatively puts forward the framework of Multi-dimensional Evidence Synthesis, Evaluation and Recommendations for TCM Interventions (MESERT). The MESERT framework is divided into three links, namely evidence generation, evidence integration and evidence translation. Based on the 4R method framework, multiple TCM efficacy research evidence (the specific method was published in the journal Frontiers in Pharmacology in 2021) was generated, and then the quantitative synthesis of randomized controlled trial (RCT) and real-world study (RWS) evidence was realized through the Bayesian hierarchical model. Finally, the MAGIC methodology system was used to integrate the research evidence with the expert knowledge system to realize the efficient and rigorous translation of research evidence to the guidelines.
MESERT framework has two core characteristics: first, it can give efficient and reliable recommendations for specific clinical problems and realize the rapid translation of TCM efficacy evidence from production to clinical practice; second, it integrates RCT and real-world evidence and achieves a comprehensive evaluation of TCM efficacy. As a supplement to the traditional guideline development method, this framework helps to promote the innovation and development of TCM efficacy evaluation and decision-making, and promote the evidence-based practice of TCM treatment.
We look forward to working with our peers in China to further improve the key production techniques of TCM efficacy guidelines and promote the translation of TCM efficacy evidence into practice by building an integrated method system for TCM efficacy evaluation and guideline recommendations.

About Key Laboratory for Real-World Data Research and Evaluation in Hainan
The Key Laboratory for Real World Data Research and Evaluation in Hainan is the first key laboratory for real world data research and evaluation in China. This key laboratory is jointly built, managed and shared by West China Hospital of Sichuan University, Boao Super Hospital and the Adverse Drug Reaction Monitoring Center of Boao Pilot Zone in Hainan Province. Based on the national strategy of building a free trade port in Hainan, and guided by the innovation of national medical product regulatory system and mechanism, the Laboratory integrates the comprehensive clinical advantages and scientific research strength of the West China Hospital of Sichuan Univeristy and the policy and platform advantages of Lecheng, Boao, trying to build a real-world data platform of “Lecheng, Hainan and China” around the approval and post-marketing regulation of innovative medical products.
Based on the overall construction mentality shifting from “based on Lecheng and covering Hainan” to “based on Hainan and covering the whole country”, the Laboratory takes Lecheng as an experimental field to innovate the real-world study practice and form a series of research fruits with the characteristics of Hainan Free Trade Port. In the future, it will play an important role in building a real-world study system leading domestically and advanced internationally and cultivating and releasing high-level, multidisciplinary talent teams and provide strong technological support for China to “take the first place in the world” in the field of real-world data applications.