2022-04-15 736
On April 11, 2022, the Hainan Institute of Real World Data published an article entitled “Evaluation of the Clinical Application Effect of the eSource Record Tools for Clinical Research” in the British journal BMC Medical Informatics and Decision Making. BMC, founded in 1999, is the world’s largest OA publisher in biomedical science, which publishes nearly 300 journals. The disciplinary scope of BMC journals covers all major fields of biology and medicine, including 57 sub-disciplines such as anesthesiology, biochemistry, and bioinformatics. The article was written by the team led by Yao Chen, vice president of Hainan Institute of Real World Data, and proposed innovative methods of the application of integrated tools for source data collection, governance and management in the conversion process from real world data to clinical research data. As a landmark achievement in the exploration made by Hainan Institute of Real World Data in the new standards, methods and tools of real-world study, the innovative method solves the bottleneck that restricts the real-world studies of imported licensed medical products in Lecheng, whose application effect has been evaluated in medical institutions in Lecheng Pilot Zone. It provides an innovative research model and valuable practical experience for guiding the real-world studies in China.

The electronic source (eSource) technology generally refers to the direct capture, collection, and storage of electronic data (such as electronic medical records (EMR), electronic health records (EHR), or wearable devices) to simplify clinical research. The eSource technology can only be fulfilled when the EMR can support research data collection.There have been some eSource-related research advances and relatively large projects in clinical trials, but the characteristics of real-world studies (RWS) require a significant investment of research costs in data collection and quality control, with limited cases and experience available for reference.
The paper reports on the clinical application effect of the eSource record tools in real-world studies. ESR tools were developed by Hainan Institute of Real World Data in cooperation with organizations such as and Hangzhou Laimai Medical Information Technology Co., Ltd., which are a new generation of integrated solutions for source data collection, governance and management of intelligent clinical researches, and can realize functions such as electronic source data collection, intelligent governance, transparent process, standardized electronic data capture (EDC) of clinical research data and remote data monitoring.
The research team selected a real-world study project of evaluating effectiveness and safety of cosmetic devices in Lecheng Pilot Zone to evaluate the ESR tools. The study used the indicators of efficiency and data transcription accuracy to compare the workflows of eSource (ESR-based source data collection and electronic transmission) and non-eSource (traditional data collection and manual transcription). Participants’ experience with ESR was assessed through the System Usability Scale (SUS) and the scales of other features (system safety, system suitability, record quality).
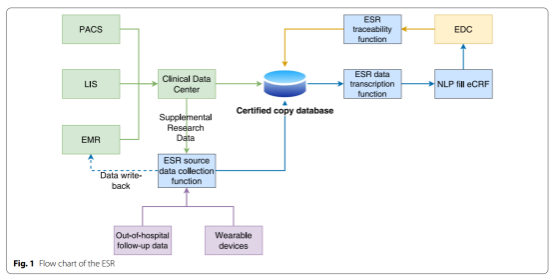
This study shows that the ESR-based eSource method can improve the efficiency of source data collection, reduce the workload of data transcription, and provide an innovative research model and valuable practical experience for guiding the development of real-world study in China.