2022-03-21 677
By Yao Chen
Editor’s note: The FDA recently cited a report saying that “80% of clinical trial data from China were ‘fraudulent or substandard’.” Yao argued that the view is outdated and misleading. He pointed out that since China joined the ICH in 2017, regulators have been introducing policies to ensure the accuracy of clinical trial data.
The FDA’s recent comments on the quality of clinical trial data from China have sparked a heated discussion in China and abroad, as its advisors mentioned that “80% of clinical trial data from China were fraudulent or substandard” in the ODAC meeting.
Innovent Biologics and Eli Lilly were seeking FDA approval for a new lung cancer drug that they co-developed based on data generated from China in the ORIENT-11 study. FDA advisors concluded that more clinical trials were needed to demonstrate its applicability to the U.S. population and medical practice.
“U.S. regulators are poised to approve dozens of cancer drugs and other new drugs developed in China,” The Wall Street Journal reported afterwards. “Regulators have expressed concern about the quality of the studies, which were largely conducted in China, and whether the results could apply to patients in the U.S.”
The FDA and Wall Street Journal reports both cited a 2016 British Medical Journal article that said “over 80% of clinical trial data submitted to support new drug registrations in China have been revealed as fraudulent or substandard by the country’s drug regulator.”
But here’s the truth: In 2015, China's drug regulatory body, formerly known as the CFDA, initiated a program that gave companies a chance to conduct self-inspections and withdraw applications without further penalty, or to provide a timeframe for making amendments to the quality of the data. By the end of 2015, 80% of companies were asked to withdraw their applications for further self-inspections.
My colleagues from Peking University and I expressed this view in BMJ in 2018. We wrote that “due to strict regulatory supervision and [the] high cost of breaking the law, deliberate data fraud is almost impossible.”
The 2015 notice from the CFDA marked a milestone in China’s regulations on clinical trials. The Center for Food and Drug Inspection (CFDI) launched a special inspection on clinical trial data and, subsequently, made regular inspections the norm for drug marketing applications. Source data verification has since then become a priority for researchers, sponsors and regulators.
In June 2017, China officially joined the ICH, becoming the eighth member in this international organization. This signified that drug regulators, drugmakers and researchers from China would be taking steps to meet international standards and follow international guidelines.
In May 2019, the NMPA issued a series of guidelines to support the use of real-world data for drug R&D and review. The Boao Lecheng pilot zone in Hainan Province has been tasked with promoting real-world data in clinical trials. In the guidelines, regulators made clear requirements for the quality and traceability of real-world source data using real-world evidence.
In September 2021, China’s National Health Commission (NHC) issued interim measures for managing investigator-initiated trials. Regulators pointed out that investigators should establish a system to store and manage source data and ensure that the clinical data are authentic, accurate, complete, confidential and up to standard when collecting, recording, modifying, processing and storing them. Investigators should also ensure data accessibility and traceability.
On Feb. 8, 2022, the NHC reiterated that health care big data is of the utmost importance. The agency is considering plans to set national standards for storing electronic medical records (EMRs) and information regarding drugs, medical devices, public health care, medical services and reimbursement in a system. The next step will be to realize data connectivity and sharing.
To further improve the accuracy of clinical data in China, my colleagues and I proposed an independent platform for synchronizing and storing research-related source data in an opinion piece in BMJ in 2018.
The platform will be primarily built and managed by the hospital, which will also be responsible for granting data access to researchers and determining the extent of data communication within the EMR system. This is to ensure that only trial-related data from subjects are synchronized and stored on the platform.
Our team worked with a health care IT industry partner in Hangzhou to develop an electronic source data recording tool called ESR. The objective was to improve the integrity of clinical trials and the safety of data collection, thereby protecting the quality of clinical trial data. Our research paper was published in the China Food and Drug Regulatory Journal (see schematic diagram). This ESR tool has also been adopted by medical institutions in the Boao Lecheng Pilot Zone and three hospitals in Beijing. The application effect remains to be seen.
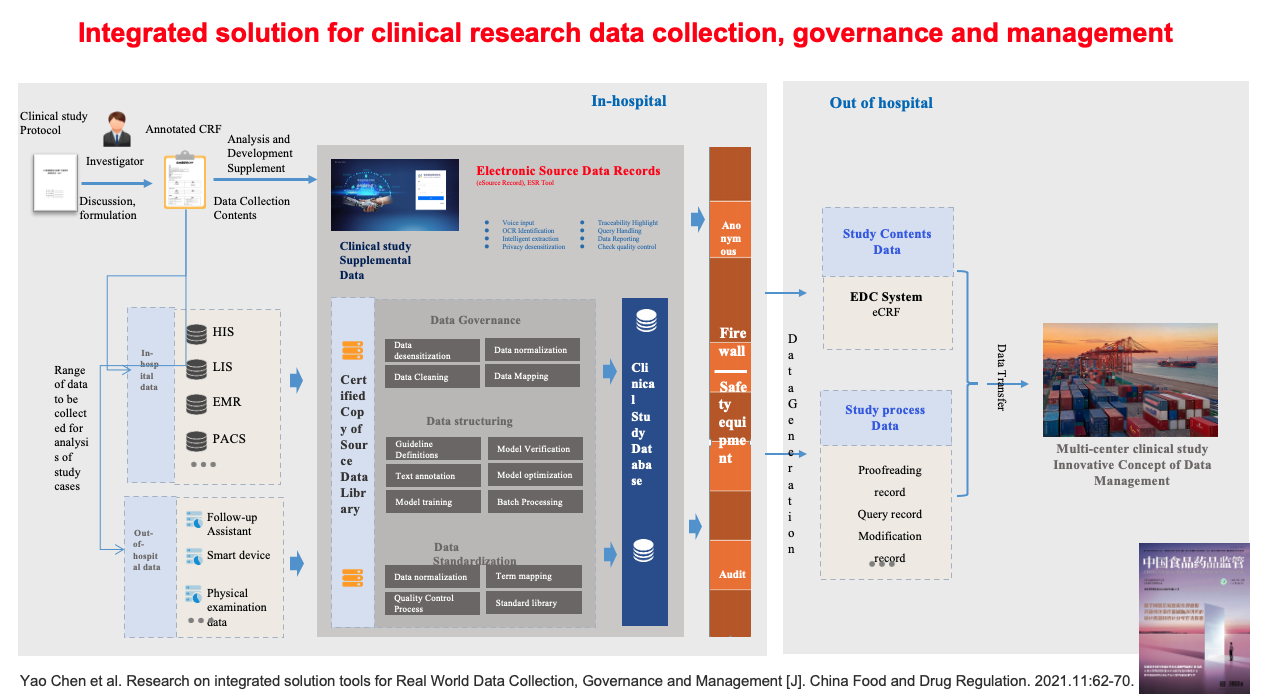
With the efforts of regulators, clinical researchers and the industry, the quality of China’s clinical trial data will continue to improve steadily.
In recent years, more new drugs developed independently by Chinese drugmakers or in collaboration with multinational partners have gone global. China is also serving as an important clinical study center, and local players are participating in many multicenter clinical trials. This says a lot about the quality of clinical trial data in China.
It may be true that issues such as the lack of representativeness of the U.S. population have prevented Innovent and Lilly from using their ORIENT-11 study to support their FDA marketing application. However, it is wrong to blame the quality of recent clinical trial data in China, especially when this view is based on a 2016 article that tells only one side of the story.
About the contributor:

Professor Yao Chen is director of the Department of Biostatistics at The First Affiliated Hospital of Peking University. He is also deputy director of the Clinical Research Institute of Peking University and deputy director of the Hainan Institute of Real-World Data. Currently, he is vice chairman of the Chinese Medical Doctor Association's evidence-based medicine committee and the Chinese Biostatistics Society's statistical theory and methods committee. He has published more than 30 papers on clinical trials and is a member of the NMPA's expert committee for evaluating new drugs and medical devices. He graduated from the Fourth Military Medical University with a degree in aviation medicine and a master’s degree in biostatistics.
source:http://www.pharmadj.com/en/cms/detail.htm?item.id=d78489bb9b7611ecbee6fa163e42049a