2022-03-05 758



On March 3, the pilot program of real-world data application of Lecheng witnessed a new breakthrough. The NMPA reviewed and approved the registration application of thermal steam treatment equipment and disposable thermal steam treatment device for prostate of Boston Scientific of the US, which were another two innovative medical devices approved based on the clinical evaluation assisted with the real-world data collected at Lecheng Pilot Zone, after the XEN Glaucoma Treatment System and Catalys precision laser system.
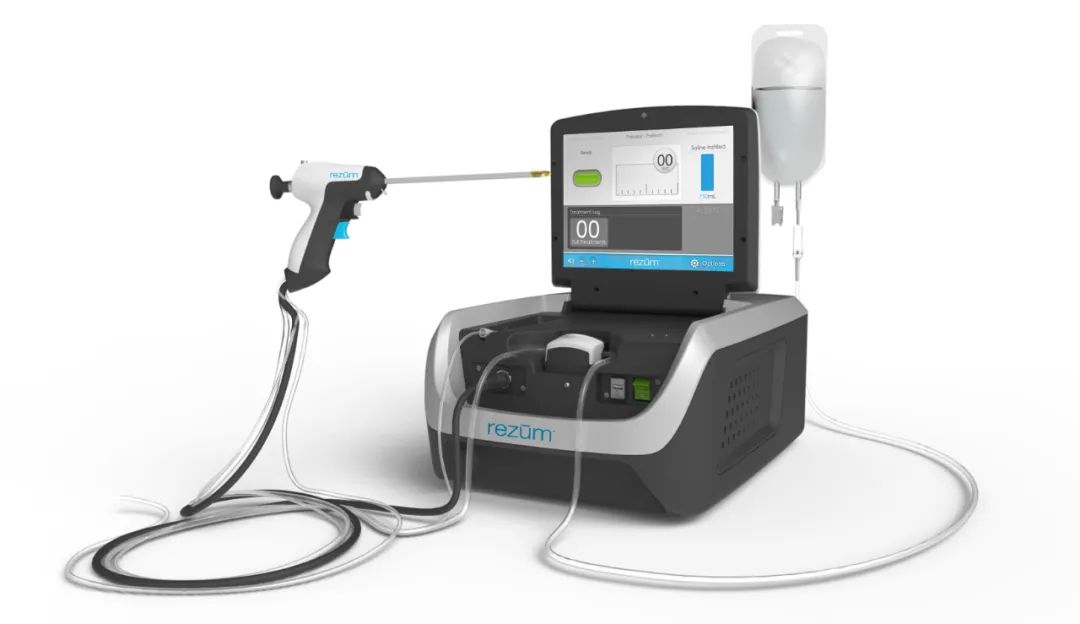
Thermal steam treatment equipment and disposable thermal steam treatment device for prostate of Boston Scientific of the US
The products use thermal steam to ablate prostate tissue to reduce prostate volume, so as to relieve obstruction and symptoms associated with benign prostatic hyperplasia (BPH), which are suitable for benign prostatic hyperplasia in men above 50 years old (inclusive) with a prostate volume of 30-80 cm3 (with or without central prostate area and / or middle lobar hyperplasia).
The products have provided a new therapy for the treatment of benign prostate hyperplasia, which is safer and more efficient than traditional surgical treatment. First, they can be performed in the operating room during the day, or as an outpatient surgery. The patients do not need systemic general anesthesia. Second, with a short time of surgery, it takes an average of 5-8 minutes only. Compared with drugs and other minimally invasive surgeries, they have a good, stable and lasting therapeutic effect, and their perioperative adverse reactions are mild. After the surgery, there is no serious sexual dysfunction. They can obviously improve the symptoms of lower urinary tract, ease the disease burden of the mid-and-old-aged patients and realize “healthy aging”.
In recent years, based on the “Double Nine Preferential Policies” of the state, the pilot program of real-world data application has been promoted at full speed at Lecheng Pilot Zone, while exploring the reform of Chinese review and approval system and accelerating the registration and marketing of global breakthrough products in China.
Riding on the piloting policy of Lecheng, these two products mentioned above achieved positive results in the treatment of Chinese BPH patients. The real-world study for these products has been finished smoothly. They have been approved only four months after the application for registration was submitted. Benefited from the special medical policy of Lecheng, near a hundred BPH patients have been treated with the innovative technology.
The pilot program of real-world data application was a major institutional innovation in Hainan Province, and a major exploration in the reform of the national drug review and approval system. The approval of the two products was an important milestone in the pilot program of real-world data application in Lecheng, which has provided the “experience of Hainan” for accelerating the accessibility of global innovative medical products in China. It was also an important practice and innovation of the NMPA in regulatory science, marking another solid step in the reform of the national medical products review and approval system, a new achievement in the construction of Hainan Free Trade Port, and a vivid embodiment of the institutional integration and integration innovation pursued by several departments together, which will attract more international advanced drug and medical device manufacturers and medical institutions to settle in Lecheng.
Zhang Jun, president of the Greater China region of Boston Scientific, said, “I am glad to see that the innovative products of Boston Scientific have been approved by real-world study for the first time. The ‘speed of Lecheng’ is so encouraging..As the medical product enterprise with the most products selected into the real-world studies in Lecheng, Boston Scientific will continue to join hands with the great health ecosphere of Lecheng and share our global R&D fruits and clinical experience to boost Lecheng’s exploration and innovation in review and approval modes and allow Chinese doctors and patients to embrace the internationally leading medical resources.”
The registration of the aforesaid products was the phased achievement of real-world studies in Lecheng, which once again showed that Lecheng has become the most important channel for international innovative medical products to enter the Chinese market. On the occasion of the the Two Sessions in 2022, the Lecheng Pilot Zone once again presented a gift to the Two Sessions with new achievements and breakthroughs.
In the future, with the progress of the real-world data application pilot program in Hainan, Lecheng will promote benchmark projects like real-world data application to “take the lead in the world”, and actively explore the application of real-world data in the registration and regulatory decision-making of medical products. It will make full use of the good opportunities from the settlement of large public hospitals such as Ruijin Hospital and West China Hospital in Lecheng to provide new solutions and schemes for the national reform of medical products review and approval system and accelerating the accessibility of global innovative products for clinical usage in China.